; Date: Tue Apr 28 2020
Tags: YouTube »»»» Coronavirus »»»»
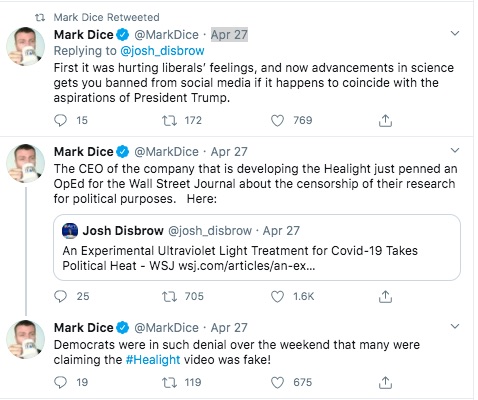
A few days ago Pres. Trump made a strange statement about using ultraviolet light inside the body as a COVID-19 treatment. At first that seemed like another of Trump's stupid ideas, but then news surfaced of a company (Aytu Bioscience) who has licensed just such a technology from Cedars-Sinai Hospital, and is rapidly trying to commercialize it. But then YouTube seems to have pulled a video from the company touting the technology, followed by a right wing agitator trying to gin up a controversy via a series of Twitter posts. The agitator is claiming this matches an existing pattern where YouTube is supposedly suppressing right wing conservatives because YouTube supposedly has a liberal bias.
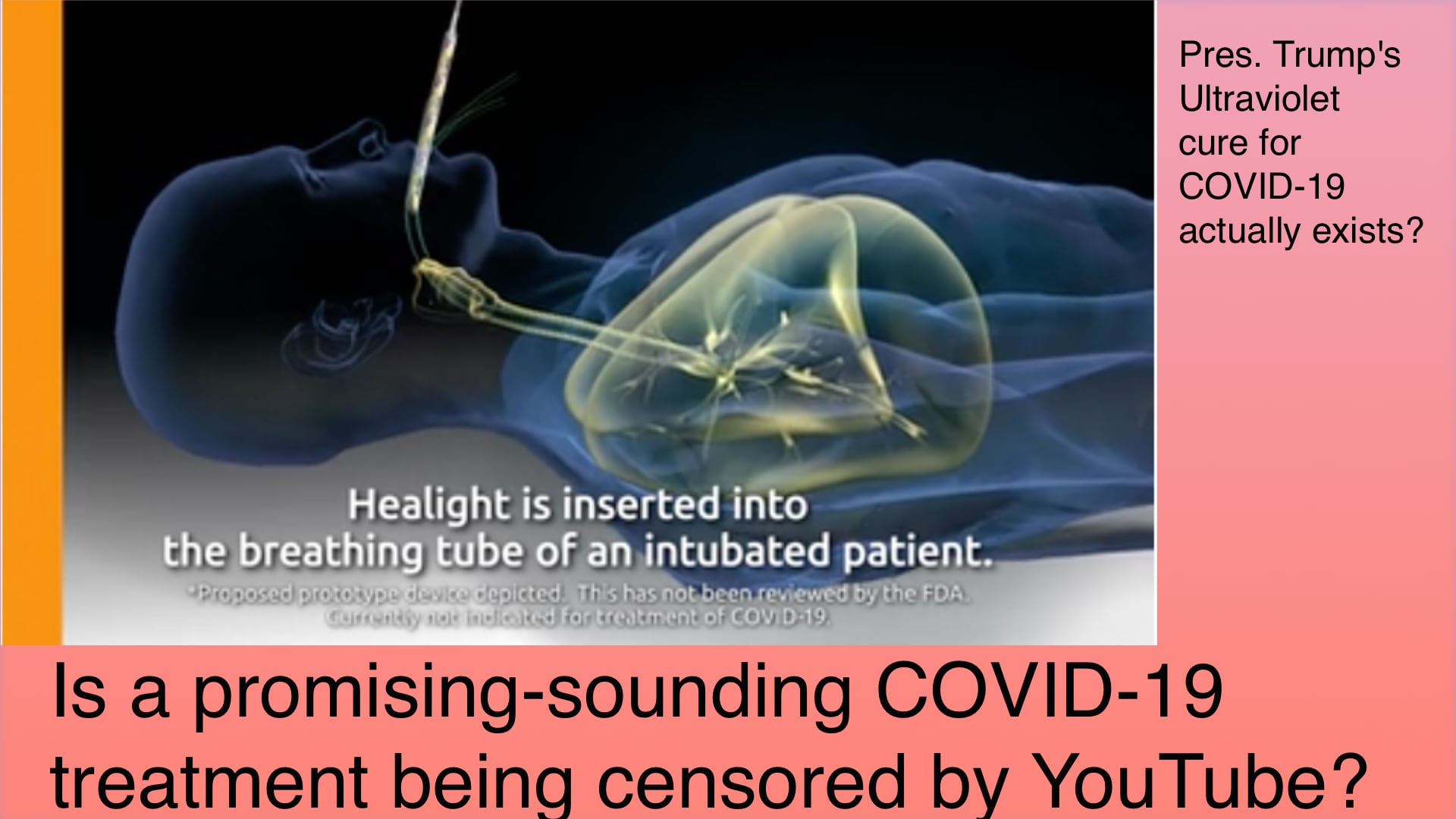
The question is whether a promising scientific advancement is being squashed by The Liberal Press just because Pres. Trump touted something like this in a press conference? That's the line of reasoning posted repeatedly on Twitter by a right wing agitator, Mark Dice.
The product - Healight - is an application of ultraviolet light that can be inserted inside a body. For the treatment of COVID-19, the plan is to insert a catheter through the breathing tube of a ventilator, and at the end of the catheter is a tiny LED that emits Ultraviolet light. The UV light is only the UV-A spectrum, the safest portion of the UV spectrum, and does not include any UV-C light which is the part of the spectrum that's known to damage genetics. The wattage of the light can be adjusted, and therefore this source of ultraviolet light can be brought into the lungs where it will kill pathogens in the lung.
In other words, this seems like an excellent treatment for COVID-19. This disease is a virus that targets the respiratory system. Ultraviolet light is widely used as a tool for disinfecting things, so therefore it's an application of the same principle inside the body. Last month we reported on a Romanian project to build an industrial robot with ultraviolet lights that could be used for disinfecting buildings. See Romanian tech lab, Modulab, develops autonomous disinfection robot amid Coronavirus worries
An important question is - how does this ultraviolet light avoid damaging useful tissue?
Yes, it is clear that ultraviolet light disinfects against pathogens. For example hospitals have autoclave devices, bins in which to disinfect tools, that use ultraviolet light. The tissues in question are surely very sensitive and not at all designed to see any kind of light. How can they prevent unwanted damage to the tissue?
I suppose we're expected to think that Cedars-Sinai Hospital is a well respected institution and would not create bogus technology. That may be. And I'm certainly not qualified to evaluate the claim. It's a question bugging in my mind.
For press releases concerning the device - see below.
Let's start with this series of tweets:
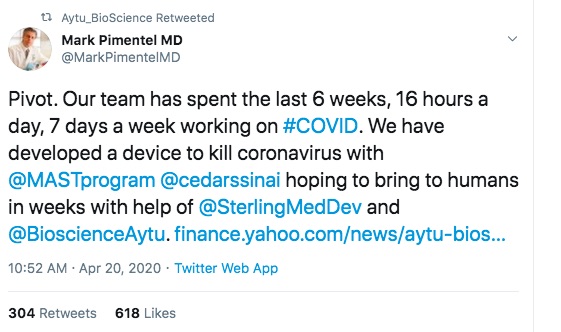
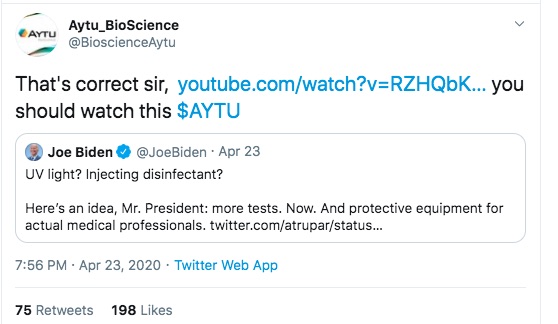
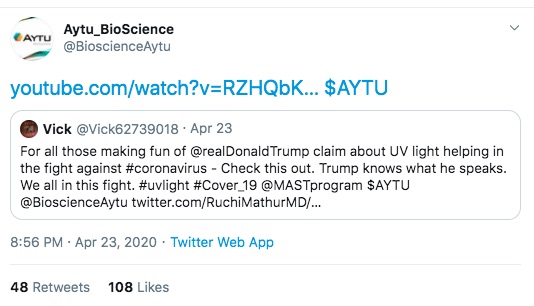
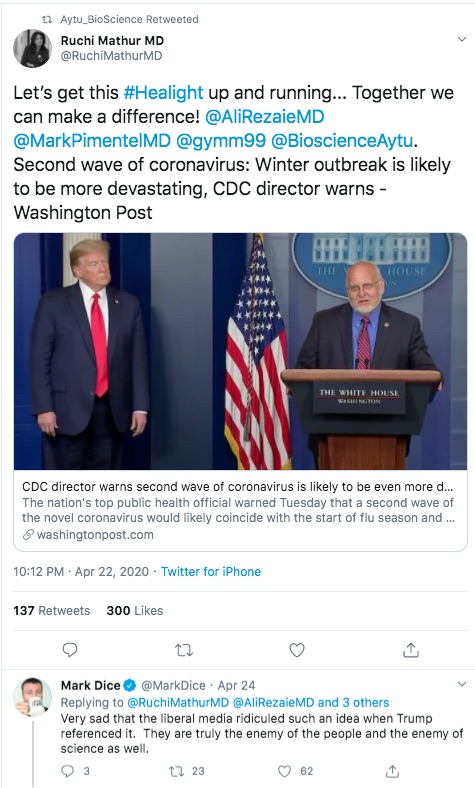
The first is one of Aytu's staff touting the product, on the day the first press release was issued. Clearly a proud product developer hoping to have a successful product launch.
The next starts to weigh in on Pres. Trump's statements during
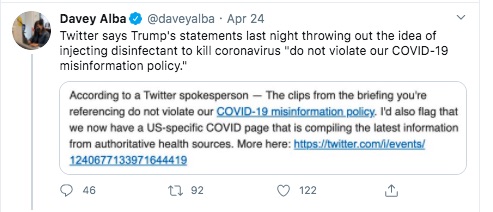
an April 23, 2020 press conference concerning current COVID-19 status. During that briefing Pres. Trump talked about using UV light internally, and talked about using "disinfectants" internally, to treat COVID-19. During the press conference, the Homeland Security Department acting Undersecretary for Science and Technology presented unpublished emerging results from a lab in Maryland about how sunlight can disinfect COVID-19 from surfaces. Then Pres. Trump mused about what if the light was brought inside the body. That idea drew a bunch of derision, and Politifact has a decent summary of what was presented. In another post,
Politifact found no evidence that UV light is administered internally.
But we're here to discuss a claim that YouTube is squashing videos posted by a promising medical products company. Some of the evidence is the tweets above.
In one tweet above you see Aytu Bioscience responding to other tweets as a way of touting their product. In one case, VP Joe Biden was calling Pres. Trump to task for talking nonsense. In other you see Aytu responding to an account with a name that suggests an automated Twitter Bot rather than a human user, again touting their YouTube video.
The problem is that YouTube video results in this:
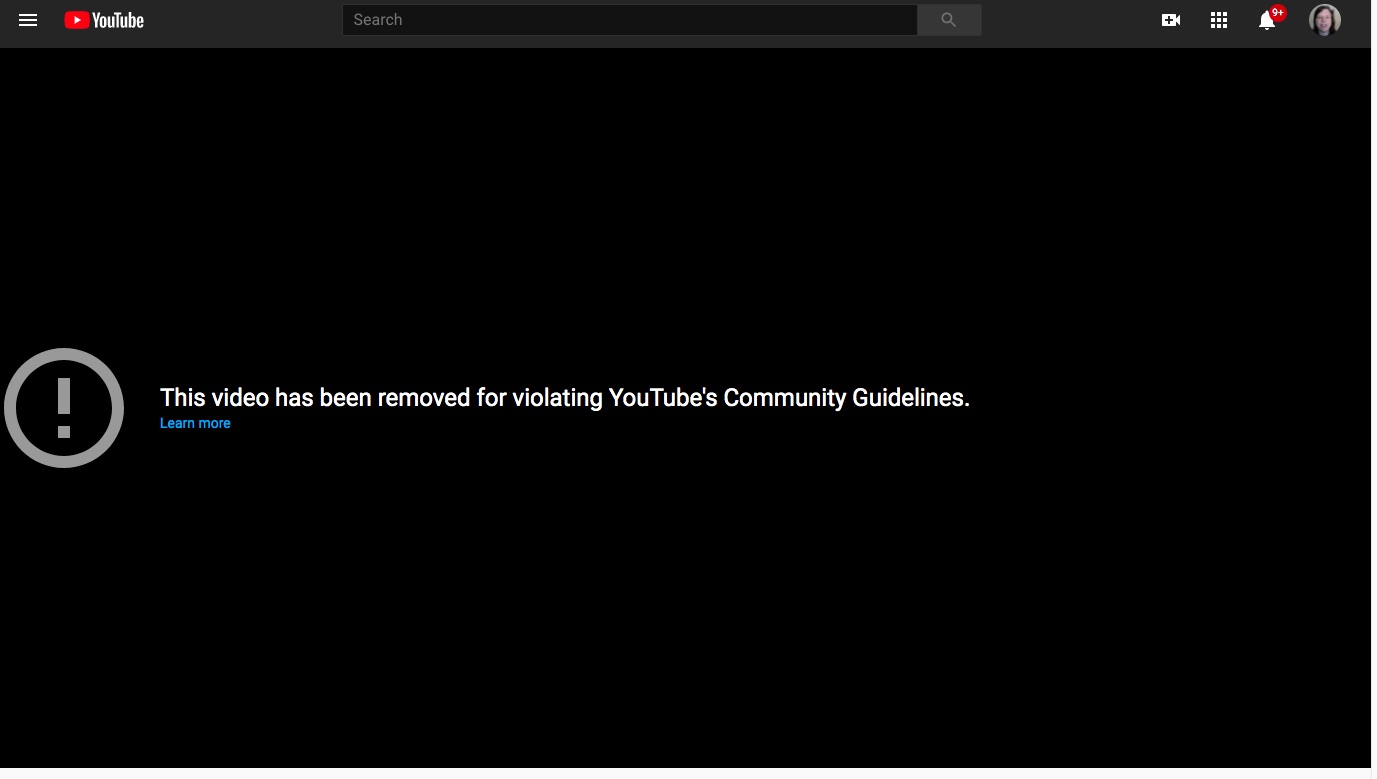
The same video is available on
Aytu's website, and is hosted on Vimeo, and seems like a benign product announcement video. There's nothing in the video that should violate any YouTube guideline. So it's curious - why is the video not available?
This is where we turn to - Mark Dice - one of the people in the above tweets. He posted a copy of the
"banned" video on Twitter. Looking at the Twitter hashtag
#Healight I see that he is the primary person posting on that hashtag, and his behavior fits the pattern of a right wing agitator. The statement above, that the liberal media is the enemy of the people, is right wing propaganda that reeks of the scary authoritarian that Pres. Trump revels in.
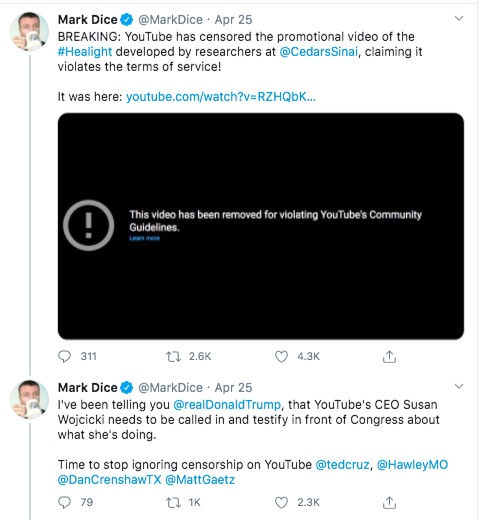
The statement that YouTube's CEO needs to be called to testify, echo's what many previous Conservative folk have claimed about YouTube. Namely, that YouTube is squashing Conservatives by suppressing their videos over fake terms of service violations.
I've covered this issue before, for example when Alex Jones (an infamous hate mongerer) was banned from YouTube, and certain right wingers made claims of Shadow Banning by YouTube and Twitter.
What's actually going on? If you look around YouTube you'll find folks from all over the spectrum (not just Conservatives) complaining about YouTube's algorithms, how YouTube is banning videos or accounts or downplaying videos or accounts or demonitizing videos or accounts. YouTube is clearly not focusing this on Conservatives, because there are plenty of people from all walks who are complaining of this. And it's not just YouTube, but also Twitter and Facebook.
At the same time of this, YouTube, Twitter, Facebook, etc, are under extreme scrutiny because of how Fake News has been gaming the system. Remember that in 2016, Russian Bots were flooding social media networks with fake news stuff aiming to manipulate public opinion and sway the USA elections. What's less recognized is that Russian Bots also attacked other elections in 2017 and 2018 in a similar way. For some background see Russia's use of Social Media channels and the Internet in Government-Government warfare
In any case, let's get back to Mark Dice:
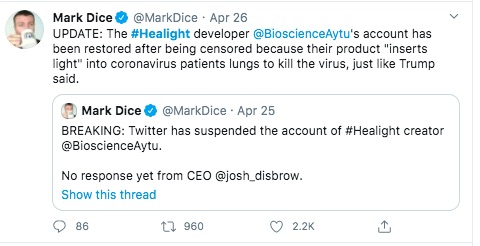
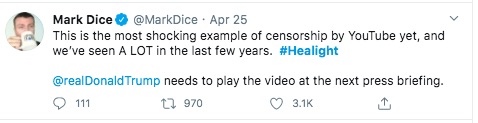
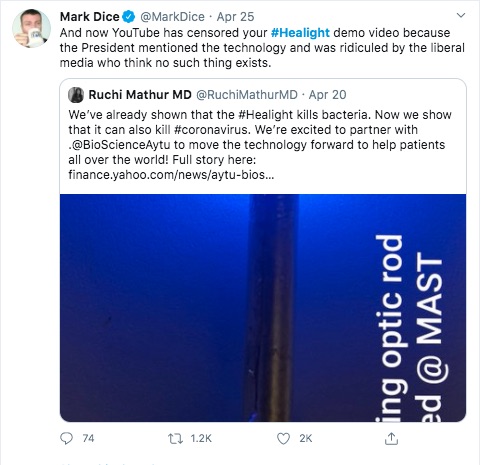
This guy has been very active pushing Aytu - and pushing the idea of Liberal Media supposedly attacking Conservatives and being an enemy of the people.
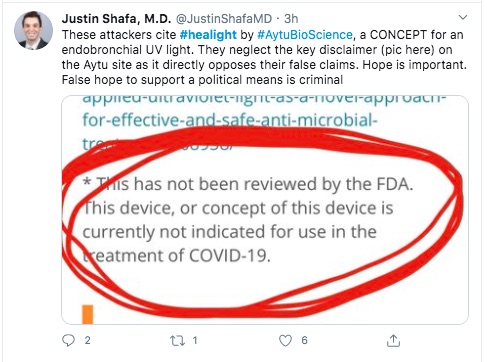
We must not lose sight of this point. The device is not properly tested, it isn't approved by anyone, etc.
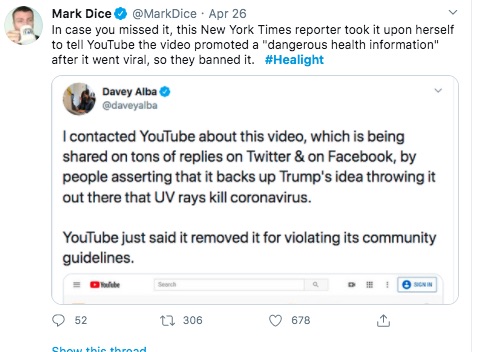
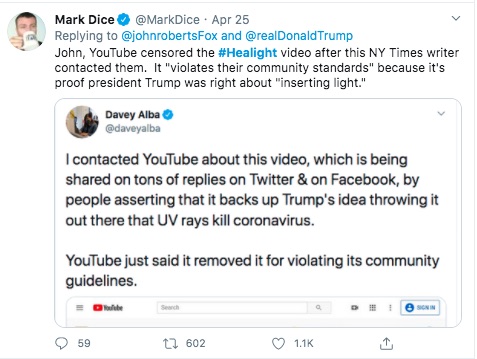
In any case - this "banning" that Mark Dice refers to, how did it happen?
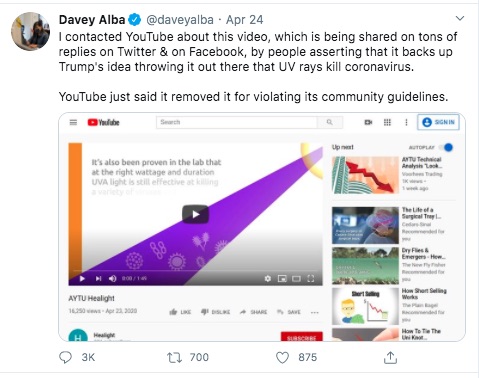
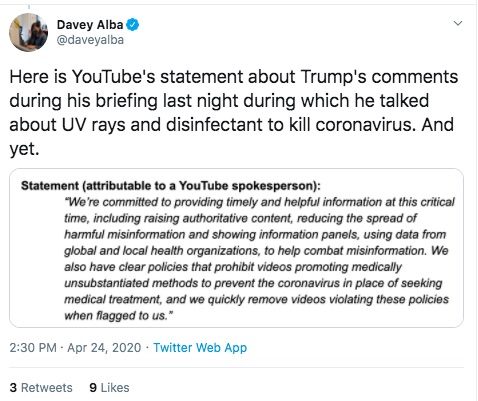
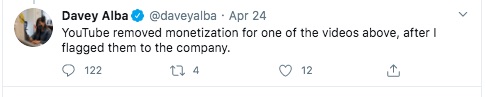

From these and other posts by Davey Alba, this person is a Journalist who cares about the accuracy of information. This person has been flagging posts to YouTube, and for some reason decided to flag the Aytu video.
I don't understand why - again, the Aytu video is a simple product announcement video. Maybe the health claims made in the video are suspicious, but I don't see how it violates any of YouTube's policies. Still, Ms. Alba flagged the video and YouTube pulled it.
A scan of YouTube's Twitter account does not turn up any explanation from them. But my understanding is that YouTube rarely explains itself when it yanks a video.
Let's get back to Mark Dice and try to figure out his angle.
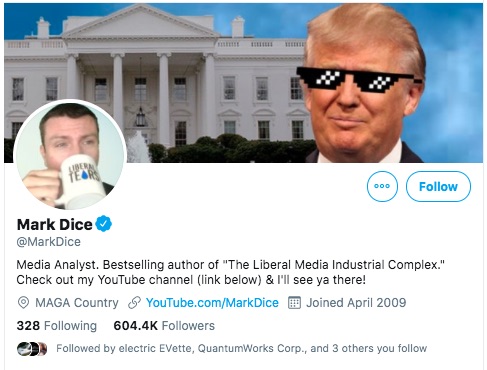
Okay - he's unabashadly Pro-Trump.
By the way, when he says the Liberal Media is the Enemy of the People, that is directly a line not just from Pres. Trump, but dates back to Nazi Germany in 1932 and before. The Nazi Party in Germany at that time frame -- one of their talking points was that the Lugenpresse was spreading lies, where they identified the normal news media (that seemingly was generally truthful) as the Lugenpresse. The word translates to English as "Lying Press." President Trump has been making similar statements as President, routinely bashing the regular news media as "Fake News Media" and attacking the press for printing what is objectively the truth.
In Nazi Germany, the Nazi Party repressed the regular press touting the Nazi-Party owned press as the outfit presenting the truth. Similarly President Trump touts the right wing media, that is generally publishing lies and falsehoods, as the real media, while bashing the regular news media as being full of liars.
In other words - Mark Dice is echo'ing a line of attack that has eery similarities to what happened in Nazi Germany.
I'm seeing a number of Twitter postings by Mark Dice talking about YouTube censorship, such as:
Doctor Erickson press conference on Coronavirus. Another attacks VP Joe Biden for supposedly being sleepy (note that Pres. Trump uses the phrase Sleepy Joe).

I don't know anything about this Dr. Erickson, but Mark Dice made several postings about him. Seems that the Dr. had one or more TV interviews claiming that
it's not effective to continue lockdown measures to fight Coronavirus, and that instead we should be going for Herd Immunity. That line of reasoning fits the Republican Right Wingers who want to reopen the economy. But the problem with that line of reasoning is the likelihood of causing a bunch of deaths.
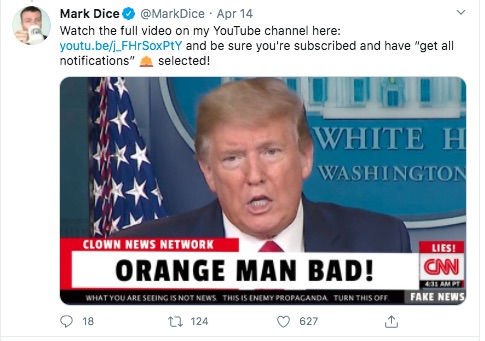
Having scrolled through a bunch of his postings - the general trend is that he's firmly in the camp of the kind of Right Wing Ideologue that unabashedly supports Pres. Trump.
This last posting fits the outline of what I described of Pres. Trump's attacks on the regular news media. This is clearly a concocted image - showing "Clown News Network" attacking Pres. Trump as the "Orange Man".
Aytu BioScience Signs Development Agreement with Sterling Medical Devices to Advance the Development of Healight as a Potential Coronavirus Treatment
Monday, April 27, 2020 9:55 AM Company has Partnered with Sterling Medical to Finalize the Development of Cedars-Sinai-Developed 'Healight' Medical Device for Use in Patients with Coronavirus
ENGLEWOOD, CO / ACCESSWIRE / April 27, 2020 / Aytu BioScience, Inc. (NASDAQ:AYTU) (the "Company"), a specialty pharmaceutical company focused on commercializing novel products that address significant patient needs announced today that it has signed an agreement with Sterling Medical Devices ("Sterling") to finalize the development of Healight, a novel endotracheal catheter, as a potential treatment for coronavirus.
The company announced last week that it licensed exclusive worldwide rights to the Healight technology from Cedars-Sinai for all endotracheal and nasopharyngeal indications. The patent-pending Healight Platform has been in development since 2016 by the Medically Associated Science and Technology (MAST) team at Cedars-Sinai. Following their pre-clinical findings that Healight may be a safe and effective antiviral and antibacterial treatment, the team engaged Sterling to rapidly develop a novel endotracheal device to help combat coronavirus.
"Sterling has been working with the Cedars-Sinai team for the past several weeks on a very accelerated schedule to develop this much needed device," said Dan Sterling, President of Sterling Medical Devices. "We are happy to now be partnering with Aytu to further advance this critical project as fast as we possibly can for the many patients in need."
"The Aytu team is very pleased to be working with Sterling Medical on this important development program and in the fight against coronavirus," stated Josh Disbrow, Chairman and CEO of Aytu BioScience. Disbrow further commented, "Sterling has a stellar reputation as a best-in-class medical device product firm with more than 21 years of experience, over 1,100 projects engineered, with none failing to receive FDA regulatory approval upon submission. Our team is actively engaged with our colleagues at Sterling in an effort to finalize the device development, with hope of enabling human use in the very near future."
The company believes the Healight platform technology has the potential to positively impact outcomes for critically ill patients infected with coronavirus and other infections. Aytu, with support of the team at Cedars-Sinai, is working with the FDA to determine an expedited regulatory process to potentially enable near-term use of the technology initially as a coronavirus intervention for critically ill intubated patients.
About Aytu BioScience, Inc.
Aytu BioScience, Inc. is a commercial-stage specialty pharmaceutical company focused on commercializing novel products that address significant patient needs. The Company currently markets a portfolio of prescription products addressing large primary care and pediatric markets. The primary care portfolio includes (i) Natesto®, the only FDA-approved nasal formulation of testosterone for men with hypogonadism (low testosterone, or "Low T"), (ii) ZolpiMist™, the only FDA-approved oral spray prescription sleep aid, and (iii) Tuzistra® XR, the only FDA-approved 12-hour codeine-based antitussive syrup. The pediatric portfolio includes (i) AcipHex® Sprinkle™, a granule formulation of rabeprazole sodium, a commonly prescribed proton pump inhibitor; (ii) Cefaclor, a second-generation cephalosporin antibiotic suspension; (iii) Karbinal® ER, an extended-release carbinoxamine (antihistamine) suspension indicated to treat numerous allergic conditions; and (iv) Poly-Vi-Flor® and Tri-Vi-Flor®, two complementary prescription fluoride-based supplement product lines containing combinations of fluoride and vitamins in various for infants and children with fluoride deficiency. Aytu recently acquired exclusive U.S. distribution rights to the COVID-19 IgG/IgM Rapid Test. This coronavirus test is a solid phase immunochromatographic assay used in the rapid, qualitative and differential detection of IgG and IgM antibodies to the 2019 Novel Coronavirus in human whole blood, serum or plasma. This point-of-care test has been validated in a 126 patient clinical trial in China and has received CE marking.
Aytu recently acquired Innovus Pharmaceuticals, a specialty pharmaceutical company commercializing, licensing and developing safe and effective consumer healthcare products designed to improve men's and women's health and vitality. Innovus commercializes over thirty-five consumer health products competing in large healthcare categories including diabetes, men's health, sexual wellness and respiratory health. The Innovus product portfolio is commercialized through direct-to-consumer marketing channels utilizing the Company's proprietary Beyond Human® marketing and sales platform.
Aytu's strategy is to continue building its portfolio of revenue-generating products, leveraging its focused commercial team and expertise to build leading brands within large therapeutic markets. For more information visit aytubio.com and visit innovuspharma.com to learn about the Company's consumer healthcare products.
About Sterling Medical Devices
Founded in 1998, Sterling Medical Devices (SMD), specializes in the product design and engineering of medical devices for the healthcare industry. Dedicated to resolving their clients' medical device design and engineering challenges, SMD addresses the whole development process, including, product design and human factors, systems, software, electronics, mechanical, quality, and compliance. The company utilizes the latest tools and technology to streamline the engineering process to speed regulatory approval of Class I, II and III devices. To date, the company has spearheaded the production of over 1,100 projects for more than 300 clients. SMD is internationally recognized and is FDA QSR 21, CFR 820, and 21 CFR Part 11 compliant, ISO 13485 registered, and IEC 62304, ISO 14971, IEC 60601, and IEC 62366 compliant. For more information, please visit www.sterlingmedicaldevices.com or call 201.227.7569 x2.
Forward-Looking Statement
This press release includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, or the Exchange Act. All statements other than statements of historical facts contained in this presentation, are forward-looking statements. Forward-looking statements are generally written in the future tense and/or are preceded by words such as ''may,'' ''will,'' ''should,'' ''forecast,'' ''could,'' ''expect,'' ''suggest,'' ''believe,'' ''estimate,'' ''continue,'' ''anticipate,'' ''intend,'' ''plan,'' or similar words, or the negatives of such terms or other variations on such terms or comparable terminology. These statements are just predictions and are subject to risks and uncertainties that could cause the actual events or results to differ materially. These risks and uncertainties include, among others: market and other conditions, the regulatory and commercial risks associated with introducing the COVID-19 Rapid Test, our ability to enforce the exclusivity provisions of the distribution agreement, the reliability of serological testing in detecting COVID-19, shipping delays and their impact on our ability to introduce the COVID-19 Rapid Test, the ability of the COVID-19 Rapid Test to accurately and reliably test for COVID-19, the manufacturer of the COVID-19 Rapid Test's ability to manufacture such testing kits on a high volume scale, manufacturing problems or delays related to the COVID-19 Rapid Test, our ability to satisfy any labelling conditions or other FDA or other regulatory conditions to sell the COVID-19 Rapid Test Kit, the demand or lack thereof for the COVID-19 Rapid Test Kit, our ability to obtain additional COVID-19 Rapid Tests to meet demand, our ability to secure additional tests if the manufacture of the COVID-19 Rapid Tests is unable to meet demand, the effects of the business combination of Aytu and the Commercial Portfolio and the recently completed merger ("Merger") with Innovus Pharmaceuticals, including the combined company's future financial condition, results of operations, strategy and plans, the ability of the combined company to realize anticipated synergies in the timeframe expected or at all, changes in capital markets and the ability of the combined company to finance operations in the manner expected, the diversion of management time on Merger-related issues and integration of the Commercial Portfolio, the ultimate timing, outcome and results of integrating the operations the Commercial Portfolio and Innovus with Aytu's existing operations, risks relating to gaining market acceptance of our products, obtaining or maintaining reimbursement by third-party payors for our prescription products, the potential future commercialization.
SOURCE: Aytu BioScience, Inc.
Aytu BioScience Signs Exclusive Global License with Cedars-Sinai for Potential Coronavirus Treatment
Monday, April 20, 2020 8:00 AM - Cedars-Sinai-Developed ‘Healight' Medical Device Platform Technology Being Studied as a Potential First-in-Class COVID-19 Treatment
Conference Call Scheduled for Tuesday, April 21, 2020 at 4:30 pm ET
ENGLEWOOD, CO / ACCESSWIRE / April 20, 2020 / Aytu BioScience, Inc. (NASDAQ:AYTU) (the "Company"), a specialty pharmaceutical company focused on commercializing novel products that address significant patient needs announced today that it has signed an exclusive worldwide license from Cedars-Sinai to develop and commercialize the Healight Platform Technology ("Healight"). This medical device technology platform, discovered and developed by scientists at Cedars-Sinai, is being studied as a potential first-in-class treatment for coronavirus and other respiratory infections.
The company will host a live conference call and webcast Tuesday, April 21, 2020 at 4:30 p.m. ET. Conference call details are provided at the end of this press release.
Led by Mark Pimentel, MD, the research team of the Medically Associated Science and Technology (MAST) Program at Cedars-Sinai has been developing the patent-pending Healight platform since 2016 and has produced a growing body of scientific evidence demonstrating pre-clinical safety and effectiveness of the technology as an antiviral and antibacterial treatment. The Healight technology employs proprietary methods of administering intermittent ultraviolet (UV) A light via a novel endotracheal medical device. Pre-clinical findings indicate the technology's significant impact on eradicating a wide range of viruses and bacteria, inclusive of coronavirus. The data have been the basis of discussions with the FDA for a near-term path to enable human use for the potential treatment of coronavirus in intubated patients in the intensive care unit (ICU). Beyond the initial pursuit of a coronavirus ICU indication, additional data suggest broader clinical applications for the technology across a range of viral and bacterial pathogens. This includes bacteria implicated in ventilator associated pneumonia (VAP).
"Our team has shown that administering a specific spectrum of UV-A light can eradicate viruses in infected human cells (including coronavirus) and bacteria in the area while preserving healthy cells," stated Dr. Pimentel of Cedars-Sinai. Ali Rezaie, MD, one of the inventors of this technology states, "Our lab at Cedars-Sinai has extensively studied the effects of this unique technology on bacteria and viruses. Based on our findings we believe this therapeutic approach has the potential to significantly impact the high morbidity and mortality of coronavirus-infected patients and patients infected with other respiratory pathogens. We are looking forward to partnering with Aytu BioScience to move this technology forward for the benefit of patients all over the world."
The company believes the Healight platform technology has the potential to positively impact outcomes for critically ill patients infected with coronavirus and severe respiratory infections. The company licensed exclusive worldwide rights to the technology from Cedars-Sinai for all endotracheal and nasopharyngeal indications. Patents have been filed by Cedars-Sinai Department of Technology Transfer, and Aytu BioScience will manage all aspects of intellectual property prosecution and filing globally. Aytu BioScience expects to partner the product outside the U.S.
"We are honored to be partnering with Cedars-Sinai as we believe the Healight therapeutic platform has the potential to help many patients during this coronavirus pandemic and beyond," said Josh Disbrow, Chairman and CEO of Aytu BioScience.
The Company is engaging with the research team at Cedars-Sinai and the FDA to determine an expedited regulatory process to potentially enable near-term use of the technology initially as a coronavirus intervention for critically ill intubated patients.
Disbrow continued, "This first-in-class technology has the potential to be a game changer for clinicians treating patients infected with coronavirus and other respiratory conditions, and our team is working tirelessly alongside the Cedars-Sinai team to determine the safety and effectiveness of this device in humans."
Conference Call Information
The company will host a live conference call at 4:30 p.m. ET Tuesday, April 21, 2020. The conference call and webcast can be accessed by dialing either number below or via the weblink:
1- 877-407-9124 (toll-free)
1-201-689-8584 (international)
https://www.webcaster4.com/Webcast/Page/2142/34401
The webcast will be accessible live and archived on Aytu BioScience's website, within the Investors section under Events & Presentations, at
aytubio.com, for 90 days.
A replay of the call will be available for fourteen days. Access the replay by calling 1-877-481-4010 (toll-free) and using the replay access code 34401.
About Aytu BioScience, Inc.
Aytu BioScience, Inc. is a commercial-stage specialty pharmaceutical company focused on commercializing novel products that address significant patient needs. The Company currently markets a portfolio of prescription products addressing large primary care and pediatric markets. The primary care portfolio includes (i) Natesto®, the only FDA-approved nasal formulation of testosterone for men with hypogonadism (low testosterone, or "Low T"), (ii) ZolpiMist™, the only FDA-approved oral spray prescription sleep aid, and (iii) Tuzistra® XR, the only FDA-approved 12-hour codeine-based antitussive syrup. The pediatric portfolio includes (i) AcipHex® Sprinkle™, a granule formulation of rabeprazole sodium, a commonly prescribed proton pump inhibitor; (ii) Cefaclor, a second-generation cephalosporin antibiotic suspension; (iii) Karbinal® ER, an extended-release carbinoxamine (antihistamine) suspension indicated to treat numerous allergic conditions; and (iv) Poly-Vi-Flor® and Tri-Vi-Flor®, two complementary prescription fluoride-based supplement product lines containing combinations of fluoride and vitamins in various for infants and children with fluoride deficiency. Aytu recently acquired exclusive U.S. distribution rights to the COVID-19 IgG/IgM Rapid Test. This coronavirus test is a solid phase immunochromatographic assay used in the rapid, qualitative and differential detection of IgG and IgM antibodies to the 2019 Novel Coronavirus in human whole blood, serum or plasma. This point-of-care test has been validated in a 126 patient clinical trial in China and has received CE marking.
Aytu recently acquired Innovus Pharmaceuticals, a specialty pharmaceutical company commercializing, licensing and developing safe and effective consumer healthcare products designed to improve men's and women's health and vitality. Innovus commercializes over thirty-five consumer health products competing in large healthcare categories including diabetes, men's health, sexual wellness and respiratory health. The Innovus product portfolio is commercialized through direct-to-consumer marketing channels utilizing the Company's proprietary Beyond Human® marketing and sales platform.
Aytu's strategy is to continue building its portfolio of revenue-generating products, leveraging its focused commercial team and expertise to build leading brands within large therapeutic markets. For more information visit
aytubio.com and visit innovuspharma.com to learn about the Company's consumer healthcare products.
Forward-Looking Statement
This press release includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, or the Exchange Act. All statements other than statements of historical facts contained in this presentation, are forward-looking statements. Forward-looking statements are generally written in the future tense and/or are preceded by words such as ''may,'' ''will,'' ''should,'' ''forecast,'' ''could,'' ''expect,'' ''suggest,'' ''believe,'' ''estimate,'' ''continue,'' ''anticipate,'' ''intend,'' ''plan,'' or similar words, or the negatives of such terms or other variations on such terms or comparable terminology. These statements are just predictions and are subject to risks and uncertainties that could cause the actual events or results to differ materially. These risks and uncertainties include, among others: our ability to successfully commercialize Healight Platform Technology, our ability to obtain FDA approval for the Healight Platform Technology, the effectiveness of the Healight Platform Technology in treating patients with COVID-19 or other illnesses, our ability to adequately protect the intellectual property associated with the Healight Platform Technology, regulatory delays, the reliability of the Healight Platform Technology in killing viruses and bacteria, market acceptance of UV based medical devices, risks associated with the COVID-19 Rapid Test including our ability to enforce the exclusivity provisions of the distribution agreement, the reliability of serological testing in detecting COVID-19, shipping delays and their impact on our ability to introduce the COVID-19 Rapid Test, the ability of the COVID-19 Rapid Test to accurately and reliably test for COVID-19, the manufacturer of the COVID-19 Rapid Test's ability to manufacture such testing kits on a high volume scale, manufacturing problems or delays related to the COVID-19 Rapid Test, our ability to satisfy any labelling conditions or other FDA or other regulatory conditions to sell the COVID-19 Rapid Test Kit, the demand or lack thereof for the COVID-19 Rapid Test Kit, our ability to obtain additional COVID-19 Rapid Tests to meet demand, our ability to secure additional tests if the manufacture of the COVID-19 Rapid Tests is unable to meet demand, the effects of the business combination of Aytu and the Commercial Portfolio and the recently completed merger ("Merger") with Innovus Pharmaceuticals, including the combined company's future financial condition, results of operations, strategy and plans, the ability of the combined company to realize anticipated synergies in the timeframe expected or at all, changes in capital markets and the ability of the combined company to finance operations in the manner expected, the diversion of management time on Merger-related issues and integration of the Commercial Portfolio, the ultimate timing, outcome and results of integrating the operations the Commercial Portfolio and Innovus with Aytu's existing operations, risks relating to gaining market acceptance of our products, obtaining or maintaining reimbursement by third-party payors for our prescription products, the potential future commercialization of our product candidates, the anticipated start dates, durations and completion dates, as well as the potential future results, of our ongoing and future clinical trials, the anticipated designs of our future clinical trials, anticipated future regulatory submissions and events, our anticipated future cash position and future events under our current and potential future collaboration. We also refer you to the risks described in ''Risk Factors'' in Part I, Item 1A of the company's Annual Report on Form 10-K and in the other reports and documents we file with the Securities and Exchange Commission from time to time.











